Medical Device Equipment Quality Assessment & Improvement Tool
Introduction
In 2012 the HIQA National Standards for Safer Better Healthcare were approved by the Minister for Health. The aim of these standards is to help drive improvements in the quality and safety of healthcare services in Ireland. Their purpose is to provide an understanding to the healthcare customer and healthcare provider alike as to what a high quality, safe healthcare service should look like. Theme 3 within these standards details the need for safe and effective management of medical devices and other equipment in accordance with legislative requirements, national policy, national guidelines where they exist and best available national and international evidence.
The Challenge
Taking account of the National Standards for Safer Better Healthcare (NSSBH) the National Medical Device Equipment Management Committee identified the need to provide a medical device equipment management assessment methodology with the aim of providing an online self assessment tool that is both reflective of the NSSBH themes and the HSE Quality Assessment & Improvement tool (QA&I). The online tool will support self assessment against the HSE “National Medical Device Equipment Management Policy” together with the supporting “Medical Device Equipment Management Best Practice Guidance for Service Areas” documents.
The Solution
The medical device equipment QA+I tool is centred on the 8 central themes together with the 27 “Essential Elements” associated with the various themes within the medical device equipment management policy and best practice guidance documents. The “Essential Elements” represent those key aspects of quality you would expect to have in place for the management of medical device equipment.
A total of 277 quality level guides are incorporated within the tool to assist in the determination of assessment on the quality level status for each “Theme” and their associated essential elements. The quality level guides represent guiding prompts that describe what a service should have in place for each level of quality.
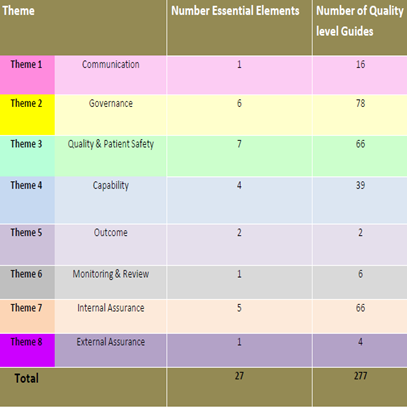
Fig 1. Assessment Structure
Levels of Quality.
For each Essential Element there are four incremental levels of quality. These levels of quality are foundation blocks which build on each other and allow services to objectively select the level of quality and maturity that most accurately reflects their service for each Essential Element. The content within each level should be viewed as guiding prompts that describe what a service should have in place for each level of quality.
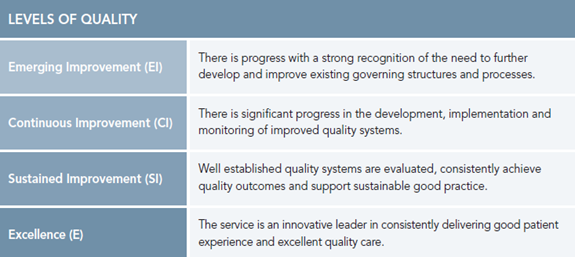
Fig 2. Levels of Quality
A guiding principle of the assessment is to create a process of continuous quality improvement progressing towards full compliance with the medical device equipment management policy and best practice guidance. Progression through the levels assumes that the main aspects within the previous levels have been achieved. Progression along this continuum also indicates that the service is maturing, becoming more sustainable and demonstrating strong leadership and innovation.
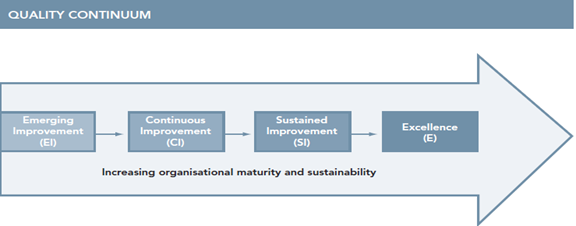
Fig 3. Quality Continuum
Quality Improvement Plan
The key output of the assessment process within the QA+I tool is the development of a quality improvement plan. Following assessment of each Essential Element, agreed actions to improve quality will be recorded in this improvement plan. The plan is then implemented and monitored through governing arrangements within each service. Following completion of the first assessment the focus will be on implementing and monitoring progress of the quality improvement plans with progress reports being developed and submitted to governing committees every quarter.
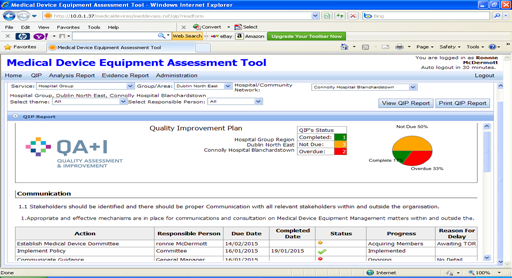
Fig 4. Sample Quality Improvement Plan.
Assessment Reports
The QA+I tool has the capacity to develop Assessment Reports for each Assessment. The report includes analysis of the results from the assessment. This analysis will illustrate the percentage of Essential Elements which achieved Emerging Improvement, Continuous Improvement, Sustained Improvement and Excellence. At a glance, assessment teams will be able to determine the areas requiring focused and targeted effort by the service.
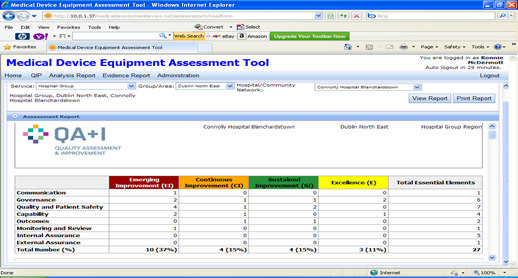
Fig 5. Sample Assessment Report.
Benefits
Use of the Medical Device Equipment QA+I tool supports a culture of improving quality across all services in relation to the management of medical device equipment. It provides opportunities for service areas to gain an informed picture of the quality of services and practices in relation to medical device equipment. The assessment process allows services to identify gaps in current service provision, develop improvement plans to address these gaps and demonstrate accomplishments achieved in the management of medical device equipment.
The completion of the medical device equipment self assessment will provide services with the supporting evidence when undertaking assessment against the HIQA "National Standard for Safer Better Healthcare" in matters pertaining to medical device equipment. The tool will support the collation of information generated from the assessment process whilst enabling the development and monitoring of any associated derived quality improvement plans to progress compliance with the HIQA standards.
- Ambulance Arrivals Project A Case Study
- SNOMED National Release Centre (NRC)
- SVUH Award winning Patient Flow Whiteboard
- Scan for Surgery
- Hospital-based care
- Digital Natives Sign App
- Digitisation of risk assesment tools for Adult mental health services in north Dublin
- Patient Engagement Operating Systems - Hep C
- Digital Transition for HSCPs at St. James's Hospital
- Primary Care Centre Castlebar Case Study
- Mario - Managing active and healthy ageing using caring service robots
- Claimsure - Health Insurance Claims Management System
- Cyber Attack Response
- Data systems in SVUH Emergency Department
- Electronic Discharge Prescription Pilot
- Epilepsy EPR
- eReferral
- eReferral Radiology Pilot
- eRostering
- Electronic Blood Tracking
- GP Practice Management Systems
- Healthmail
- Heart Failure Virtual Clinic
- Infrastructure - MPUP to ECAM
- IT Security - Small changes, big difference
- Kidney Disease Clinical Patient Management System
- Local Asset Mapping Project at St James' Hospital
- LUCY
- Mi Kidney App
- Model Community
- NCHD - Employment Record Portal
- Nursing & Midwifery Quality Care Metrics
- Ophthalmology Electronic Patient Record
- PharmaBuddy
- Radiology & Electronic Patient Record
- National Smart-Pump Drug Library of Paediatric and Neonatal Standardised Concentration Infusions
- Quality & Patient Safety
- Robotic Assisted Surgery Programme
- Shared Learning on EHR
- St. James' Hospital - National Haemophilia System
- Tallaght Hospital Pharmacy
- Tallaght Hospital Patient Engagement App
- Track & Trace
- Using IT to Improve Ireland's Public Sector Healthcare
- National Audiology Clinical Management System (NA-CMS)
- St Vincent's University Hospital Award Winning Whiteboard Patient Flow System
- Snomed Case Study
- Telehealth Project Donegal
- St Vincent's Whiteboard Patient Journey System a Case Study
- Ambulance Arrivals Project
