Electronic Blood Tracking System (EBTS)
 Introduction
Introduction
Over 1,000 people receive transfusions every week in Ireland. This represents a substantial amount of blood movement from the Irish Blood Transfusion Service (IBTS) to the hospitals.
The need for EU and national laws covering the safety of blood transfusion arose out of the bleak events of the 1980s and 1990s where poor transfusion practices in several countries including Ireland, contributed to the spread of HIV and hepatitis in Europe and throughout the world. Creutzfeldt-Jakob Disease (CJD) has also contributed significantly to the problem as currently there is no test for donors. Consequently it is necessary to have complete traceability for all blood products due to the long incubation period of the CJD prion EU Blood Directive 2002/98/EC enacted in Statutory Instrument 360 of 2005 seeks to ensure that the best practices in transfusion are adopted and available everywhere in Europe by “setting standards of quality and safety for the collection, testing, processing, storage and distribution of human blood and blood components” and covers both blood establishments and hospital blood banks. The effective date was November 2005, however the Irish government granted hospital blood banks a three year derogation for the quality system and they had to be fully compliant by November 2008. By the November 2008 deadline hospital blood banks achieved compliance using paper-based traceability systems with some hospitals further supported by electronic blood tracking systems.
The Health Service Executive's (HSE) vision or goal in terms of blood administration is to be a world leader by combining the use of information technology combined with international best practice to administer blood products to the right patient at the right time whilst recording all relevant data including serious adverse reactions and serious adverse events.
This case study outlines the improvements brought about by the introduction of an Electronic Blood Tracking system for Ireland.
The Challenge
The following work flow diagram shows the typical movement associated with 99% of blood that is issued and managed at hospital level and applies irrespective of whether the resulting data is recorded manually on a paper-based system or on an electronic blood tracking system.
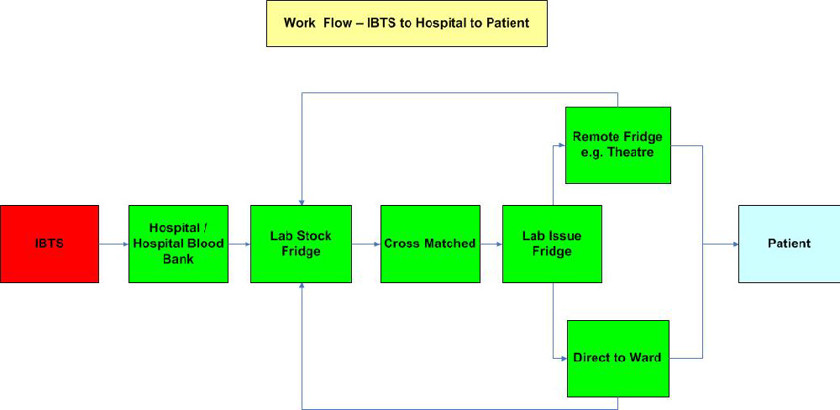
Initially blood is dispatched by the IBTS to the hospital/hospital blood bank. It must be maintained in a controlled environment which is dependent on the blood product e.g. red cells must be maintained in an environment where the temperature is 4ºC +/- 2ºC, platelets must be stored at room temperature i.e. 22 ºC +/- 2ºC on a controlled agitator. When the blood/blood product arrives at the receiving destination it is logged into the stock fridge in the laboratory. Thereafter it can be cross matched for a patient and when ready for issue is placed in the issue fridge. It is then collected by a porter or a nurse and taken to a remote fridge or directly to the ward for the patient. It must be tracked at all stages during its movement i.e. stock fridge to issue fridge; issue fridge to remote fridge; issue fridge to ward; remote fridge back to stock fridge etc. Additionally a record must be maintained on the length of time spent outside the controlled environment as unused units of red blood cells or whole blood must be returned to the blood bank within 30 minutes of the issue time, otherwise they will be discarded. Frequently blood is cross matched for a patient but is not subsequently used. This blood can be cross matched for multiple patients during the course of its shelf life provided it does not exceed the 30 minute out of fridge rule.
All of the above blood movements must be tracked so as to ensure that this scarce and costly life-saving product is used as efficiently as possible. While the paper-based traceability system ensures compliance with the EU Blood Directive, clearly there are many difficulties with it including:
- No accurate data on how long the blood/blood product is out of the issue fridge which in turn is contributing to inefficiencies and waste of a scarce and costly life-saving product.
- The amount of transcribing involved in a paper-based system increases the risk of transcription error. All such errors are termed a non-conformance and must be managed accordingly. These errors are likely to increase further when the IBTS changes from the current 7-digit Codabar to the 14-digit ISBT 128.
- Inefficiencies due to the amount of duplication (transcription).
- Substantial resources required to follow-up the paper trail on an on-going basis.
- Involves the storage and retrieval of records from a paper-base with the requirement to retain all records for 30 years.
- A paper-based traceability system cannot support compliance in the same way as an electronic blood tracking system e.g. it will provide step-by-step instructions through the blood process workflow and will have built-in warnings to prevent user error etc.
There was increasing demand for an electronic blood tracking system (EBTS) to replace the paper-based traceability system. Additionally it offered opportunities to manage blood stock management in a more efficient manner.
All of these factors lead to the establishment of the EBTS Project.
The Solution
The EBTS Project is subject to peer review. This is an independent, structured review of a programme or a project carried out at key decision points by a team of experienced people made at the request of IT Control within the Centre for Management and Organisation Development (CMOD), now known as the Office of the Government Chief Information Officer (OGCIO). To date the EBTS Project has been reviewed at the following stages:
-
Business Case
-
Request for Tender (RFT) (before the document is published)
-
Tender Evaluation (before the preferred supplier is notified)
-
Contract Review (before the contract is signed)
-
Project Initiation Report
-
Checkpoint Review (to review the Phase 1 rollout and to endorse decision to proceed to get approval and funding for the Phase 2 rollout)
-
Checkpoint Review (to review the Phase 2 rollout and to endorse decision to proceed to get approval and funding for the Phase 3 rollout).
Further Checkpoint Reviews may be requested and it will be necessary to undertake a Post Implementation Review when the EBTS work programme is completed.
The EBTS Project Team with assistance from the EBTS Procurement Group carried out the procurement following the EU restricted route. Its objective was to procure a functionally rich ‘off the shelf’ EBTS, the scope of which includes the:
-
Supply
-
Installation and Acceptance Testing
-
Configuration
-
Integration including interaction with third party vendors, delivery of all interface requirements with associated costs
-
Delivery of the entire training programme
-
Implementation
-
On-going support on a 24 x 7 x 365 basis.

The HSE signed the contract with Accuscience Ireland Ltd on 12-July-2012 to supply BloodTrack® Blood Management and Bedside Transfusion Solutions (BloodTrack). The EBTS Project is a three phase work programme. The official commencement date for the Phase 1 rollout was 14-January-2013. The initial approval received by the EBTS Project Team was for Phase 1 rollout only. It was necessary to seek approval including funding for each of the three phases separately.
The following diagram provides a description for each of the three phases of the EBTS work programme:
|
EBTS Project – Proposed Rollout for HSE |
|||||
|
Phase |
Areas Included |
HSE Staff Affected |
Number of HSE Locations |
Interface Required |
HSE Deadline for Completion |
|
|
Track blood component/product into and out of refrigerators |
Medical Lab Scientists Porters Nurses Haemovigilance |
48 hospitals |
Laboratory Information System (LIS) |
Within 10 months of the agreed commencement date |
|
Record arrival of blood component/product in high volume usage areas and record fate of unit |
Nurses Doctors Haemovigilance |
ICU & theatre in 48 hospitals |
|||
|
Maintain current paper-based traceability systems in all other areas |
Nurses Doctors Medical Lab Scientists Haemovigilance |
All hospital departments except ICU & theatre |
|||
|
|
Record arrival of blood component/product on ward and record fate of unit |
Nurses Doctors Haemovigilance |
All remaining hospital departments i.e. locations not completed during Phase 1 |
LIS |
Within 2 years of the agreed commencement date |
|
|
Record all blood related events at the patient’s bedside i.e. from transfusion sample to fate of unit |
Nurses Doctors Haemovigilance |
All 48 hospitals |
HIS |
Within 5 years of the agreed commencement date |
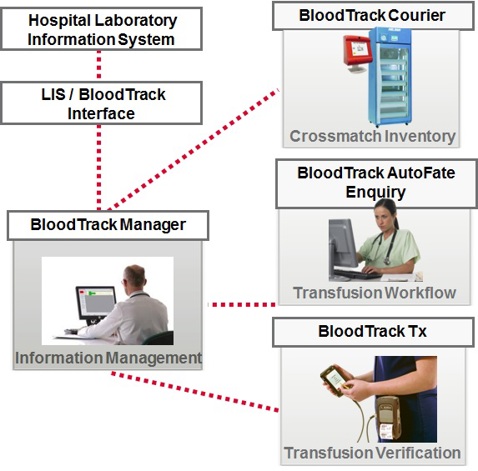
The key points relating to the proposed modular rollout of an electronic blood tracking system in the HSE include:
Phase 1:
a) Electronic tracking of blood component/product into and out of refrigerators in all 48 HSE hospitals
b) Recording arrival of blood component/product on ward and recording fate of unit by healthcare professionals in high volume usage areas i.e. theatre & ICU. This task will be completed retrospectively at the nurse’s workstation or other designated workstation.
c) The current paper-based traceability systems will be maintained in all other areas.
d) An interface to the LIS will be required in order to facilitate the seamless exchange of information between the two systems
e) Hospitals will go live on a site by site basis culminating in all 48 HSE hospitals live with Phase 1 of the EBTS within 10 months of the agreed commencement date.
Phase 2:
a) Extending the rollout of ‘recording arrival of blood component/product on ward and recording fate of unit by healthcare professionals’ to all remaining hospital departments and phasing out the current paper-based traceability systems. This task will be completed retrospectively at the nurse’s workstation or other designated workstation.
b) The interface to the LIS will be in place from phase 1 of the project.
Phase 3:
a) Recording all blood related events using a PDA (personal digital assistant or handheld PC) at the patient’s bedside. Blood related events include all transactions from taking the transfusion sample to fating the unit at the patient’s bedside initially in high volume usage areas i.e. theatre & ICU
b) Recording all blood related events using a PDA at the patient’s bedside. Blood related events include all transactions from taking the transfusion sample to fating the unit at the patient’s bedside in all remaining hospital departments.
c) An interface to the HIS will be required in order to facilitate the seamless exchange of information between the two systems.
The rollout of phases 2 and 3 of the EBTS Project is dependent on:
- HSE, Department of Health and Department of Finance approval and funding for phases 2 and 3 of the work programme.
- The successful deployment of BloodTrack as part of the phase 1 and 2 work programme. This is determined by a formal project review that encompasses organisation, product and vendor performance and is completed at the end of phase 1 and 2.
The EBTS Project has appropriate project governance in place including:
- Project Sponsors – Mr. Fran Thompson and Mr. Gerry O’Dwyer
- Project Board – chaired by Mr. Gerry O’Dwyer
- Procurement Group – chaired by Mr. Mike O’Regan. It remained in place up to the time of completing the procurement process.
- Project Team – Mr. Denis Maher, ICT Project Manager, Mr. Tony Finch, Business Manager, Ms. Catherine McEvoy, Project Analyst, Mr. Chris Plunkett, Infrastructure & Operations (input as required), Mr. Peter Mannion, Citrix (input as required), Ms. Norma O’Brien, Haemovigilance (input as required)
- Hospital Implementation Groups (HIGs) – all hospitals
- Network Working Groups
To date all hospitals with the exception of Cavan General Hospital have implemented Phases 1 and 2 of the EBTS work programme. Cavan General Hospital is an existing BloodTrack site and will join the national system following a software upgrade that is planned for release during Q3 2015 thus allowing them maintain some existing functionality. Currently Phase 3 is being rolled out across the HSE. This is the phase that is most eagerly awaited by staff as it brings BloodTrack to the patient’s bedside using a PDA. All blood related events from taking the initial blood sample for cross match to completing the final fate of unit is completed at the patient’s bedside using a PDA. The EBTS Project remains on target and will be completed within five years of the official commencement date of 14-January-2013.
The Process
The EBTS Project Team works closely with Accuscience to rollout the BloodTrack system. Each hospital has a nominated EBTS Lead who is the main point of contact between the hospital and the EBTS Project Team. The EBTS Lead operates on an ‘as required’ basis and co-ordinates all work ensuring that all tasks/dates/changes/schedules are agreed with representatives on the Hospital Implementation Group (HIG). All hospitals have a HIG and their contribution to the EBTS Project is also on an ‘as required’ basis. In addition to ensuring that the broader requirements of the EU Blood Directive are in place for their respective hospitals, the HIG implements the BloodTrack system at hospital level. However it is the CEO/Hospital General Manager who is ultimately responsible for the implementation of the EU Blood Directive and in due course, the implementation of the EBTS in his/her respective hospital. The HIG is drawn from the following staff groups:
- Hospital management
- Clinical Director
- Haematologist
- Laboratory
- Haemovigilance
- Risk management
- Nursing
- ICT
- Estates
- Porters.
Detailed site surveys are completed in advance of commencing each phase of the EBTS work programme to determine the state of readiness and to provide each hospital with a detailed breakdown on dependencies that must be addressed in order to support the BloodTrack rollout. The range of dependencies for Phase 1 ranged from LIS upgrades in order to support the LIS interface to BloodTrack, provision of barcoded user ID badges, installation of network and power points to support the BloodTrack hardware. The main dependencies that require addressing for Phase 3 are the provision of 2D barcoded compatibility labels, 2D barcoded patient wristbands and a wireless network. The Project team recommend wireless but will work with hospitals and provide a wired solution i.e. where the PDA is docked by a USB cradle via a ward PC.
The Project Team acknowledges the significant input made by all hospitals to date in rolling out the BloodTrack system.
The Results
To date the EBTS Project remains on target to be completed within five years of its official commencement date of 14-January-2013.
The project offers many benefits both in the short and longer term covering such areas as safety, increased efficiencies and cost savings. Some of these benefits will be evident from an early stage in the implementation process whereas others will not be realised until all three phases of the EBTS work programme are complete.
The following provides a sample of the benefits from implementing the BloodTrack system:
Patient Benefits:
- Increases public confidence and trust in our hospital-based blood traceability system.
- Assurance can be provided on traceability as the blood product will be tracked at all stages in the hospital environment.
- Assurance can be provided on product quality due to the built-in safety mechanisms e.g. the user will be warned if the blood product is outside of its controlled environment (i.e. fridge) longer than is permitted etc.
- Patient safety will be upheld and protected at all times i.e. right patient receives the right blood.
- Reduce the need to re-take blood samples for compatibility testing resulting from inaccurate, incomplete or illegible labelling. Printing the label at the patient’s bedside will support this requirement from Phase 3.
- Reduce the median time to deliver urgently required blood products to patients from time of request. Electronic release of blood products from electronically controlled issue fridges will support this requirement from Phase 3.
- Prevents the transfusion of incorrectly stored units as:
- Out of date product or quarantined products will not be released by BloodTrack.
- Minimise the risk of transfusing units that have been out of controlled storage for too long (>30 minutes).
Organisational Benefits:
- Assists staff achieve compliance with EU Blood Directive and provides support for accreditation and quality initiatives e.g. ISO 15189.
- BloodTrack provides a single standardised system to all HSE employees thereby facilitating staff movement from hospital to hospital and a subsequent reduction in errors due to differing systems at hospital sites.
- Achieves greater efficiencies in the blood transfusion process e.g. reduce blood wastage.
- Increases overall accountability and confidence in the blood services i.e. vein to vein traceability.
- Provides a full audit trail of all activity.
- Staff can quickly and efficiently scan blood products from location to location thus reducing the risk of transcription errors.
- Improves the overall clinical transfusion process by integrating relevant standard operating procedures e.g. provide step-by-step instructions on blood administration.
- Provides potential to revolutionise the workflow within the hospital blood banks/hospitals i.e. provides remote release of blood.
- Frees up nursing resources to allow them concentrate on other core nursing duties resulting from one nurse required instead of two to administer the blood product, less re-working i.e. first-time right and time taken to check transfusion is reduced.
- All adverse events associated with blood product delivery to patients will be captured and logged on the system.
- Provides a tool to manage unnecessary transfusion of product as it:
- Facilitates quality systems and audit of blood usage for departments, units, individuals etc.
- Facilitates monitoring of transfusion units and appropriate management of therapeutic dose requirement.
Exchequer Benefits:
- Reduces patient risk with associated cost savings.
- Reduces risk of litigation.
- Reduces blood wastage throughout the hospital.
- Frees up nursing resources allowing them concentrate on other core nursing duties.
It was estimated that there would be savings of €810,000 following the rollout of Phases 1, 2 and 3 resulting directly from blood product with a further saving of €2,250,000 on Whole Time Equivalents (WTEs). However the latter will not be realised in monetary terms. Instead nursing time will be freed up thus allowing nursing staff concentrate on core nursing duties.
To date BloodTrack has assisted with blood stock management and has seen red blood cells outdating reduce from a figure of > 10% at a cost of over €3 million to 0.8% in 2014 at a cost of €0.22 million. BloodTrack also supports re-routing of red blood cells. In 2014 3,558 units were re-routed resulting in a saving of €0.89 million. 98.5% of all re-routed red cell packs were transfused. There is a similar process in place for platelet stock management, again supported by BloodTrack. In 2010 there was >10% outdating and this is now reduced to 4.78% in 2014.
Next Steps
The Project Team and the supplier will continue the rollout of BloodTrack to the patient’s bedside and will do so with the help and co-operation of the EBTS Leads and HIGs. BloodTrack should be fully rolled out to all locations by Q4 2016/Q1 2017.
- Ambulance Arrivals Project A Case Study
- SNOMED National Release Centre (NRC)
- SVUH Award winning Patient Flow Whiteboard
- Scan for Surgery
- Hospital-based care
- Digital Natives Sign App
- Digitisation of risk assesment tools for Adult mental health services in north Dublin
- Patient Engagement Operating Systems - Hep C
- Digital Transition for HSCPs at St. James's Hospital
- Primary Care Centre Castlebar Case Study
- Mario - Managing active and healthy ageing using caring service robots
- Claimsure - Health Insurance Claims Management System
- Cyber Attack Response
- Data systems in SVUH Emergency Department
- Electronic Discharge Prescription Pilot
- Epilepsy EPR
- eReferral
- eReferral Radiology Pilot
- eRostering
- Electronic Blood Tracking
- GP Practice Management Systems
- Healthmail
- Heart Failure Virtual Clinic
- Infrastructure - MPUP to ECAM
- IT Security - Small changes, big difference
- Kidney Disease Clinical Patient Management System
- Local Asset Mapping Project at St James' Hospital
- LUCY
- Mi Kidney App
- Model Community
- NCHD - Employment Record Portal
- Nursing & Midwifery Quality Care Metrics
- Ophthalmology Electronic Patient Record
- PharmaBuddy
- Radiology & Electronic Patient Record
- National Smart-Pump Drug Library of Paediatric and Neonatal Standardised Concentration Infusions
- Quality & Patient Safety
- Robotic Assisted Surgery Programme
- Shared Learning on EHR
- St. James' Hospital - National Haemophilia System
- Tallaght Hospital Pharmacy
- Tallaght Hospital Patient Engagement App
- Track & Trace
- Using IT to Improve Ireland's Public Sector Healthcare
- National Audiology Clinical Management System (NA-CMS)
- St Vincent's University Hospital Award Winning Whiteboard Patient Flow System
- Snomed Case Study
- Telehealth Project Donegal
- St Vincent's Whiteboard Patient Journey System a Case Study
- Ambulance Arrivals Project

