HSE National Medical Device Alert System
Introduction
The Health Products Regulatory Authority (HPRA) is an independent public sector organisation who are the competent authority for the regulation of health products including medical devices and equipment used for medical purposes within Ireland.
The HPRA publishes notices relating to the safety and/or quality of medical devices. The issues covered by these notices range from quality defect information to product recalls, to updated information on the appropriate use of the medical devices. The majority of these notices are for the attention of health professionals including those working in hospitals, community healthcare organisations and other health facilities.
The recipients of the safety communication have a responsibility to ensure that the communication reaches the most appropriate personnel within their organisation and to ensure that the issue outlined in the notice is considered, the risks assessed and the appropriate / recommended actions are completed.
The Challenge
The HSE is responsible for ensuring that a Medical Device Safety Notice and Hazard Alert notification process is operational. On receipt of HPRA notifications it is the responsibility of the HSE to ascertain if the safety notice or alert pertains to any of the locations within the HSE or HSE funded voluntary services. Following on from the publication of the HSE’s Medical Device/Equipment Management Policy (2009) it was recognised that a set of standard processes and a supporting ICT application is required in the HSE to manage the Medical Device Safety Notice and Hazard Alert notifications. It was also recognised that a suite of reports is required to assist measuring the activity and management of both alerts and notifications.
Objectives
- Provide a national ICT system to manage Medical Device Safety Notices and Hazard Alerts pertaining to any of the locations within the HSE or HSE funded voluntary services
- Provide an easy and efficient method of monitoring the status of responses to safety notices and alerts facilitating adherence to critical timelines via electronic reminders and alerts
- Provide a central data repository from which statistical and management reports can be extracted.
The Solution
The general structure of the system is as illustrated in Fig 1:
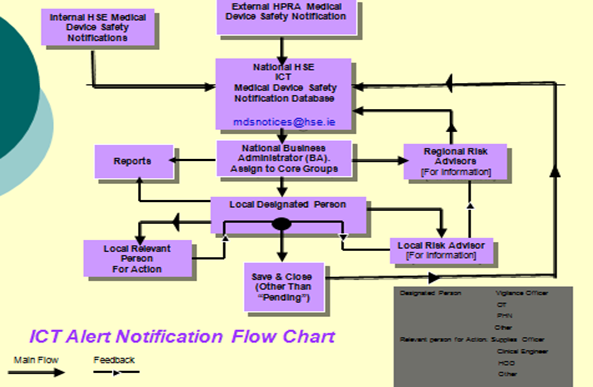
Fig 1. System Outline
- The system receive notification directly from the HPRA of all Medical Device Safety / Hazard notifications or any internal generated HSE safety notifications for distribution. A priority level is assigned to each alert in accordance with the HPRA “Traffic Light System” of red, amber and green. An associated automated response timescale is also assigned to each notification within which the relevant action must be reported back by the “Designated Person” to the Central ICT System as being “Completed” or “Not Applicable” or “Other”.
- A notification is generated to the relevant “Designated Persons” via their HSE email account of an alert for processing within an appropriate timescale
- The Designated Person will log into the system and review the relevant notification for processing. If applicable the alert will be forwarded on to the relevant person/s within their organisation for action.
- The designated person will choose one of the predetermined options listed for “Action Taken” on the system to close off the alert response details for the national system
- Where actions taken are not listed in the drop down menu for the predetermined response options the “Designated Person” can detail their specific actions taken within a comments box.
- Automated reminders will be issued from the national system to “Designated Persons” who have not closed off alerts received from the national system:
- The National Business Administrator will have view within the national system of the responses received from the designated person/s together with ability to generate Hospital Group performance reports for CEO consideration.
Benefits
The system is presently operational to provide Local, Area, Group and corporate assurance both in the Acute and Community Healthcare Organisations for the management of medical device alerts as distributed by the HPRA and others.
Local and corporate assurance is delivered by way of delivering a form of a “Closed Loop” system by assigning to the national ICT system nominated “Designated Persons” within each service facility to take responsibility for the receipt of the medical device alert notifications. The “Designated Person” will ensure the further internal facility distribution to the relevant personnel for implementation of the recommended actions where applicable. The notice is automatically closed on the national system when the “Designated Person” has completed the “Response Details” screen and where notices are not closed an automatic reminder email is generated.
“Closed Loop” System
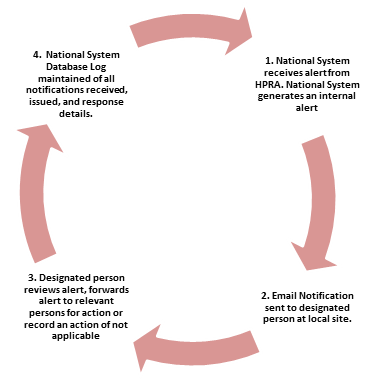
Other benefits include:
- Standardised processes for handling Medical Device Safety Notices and Hazard Alerts within the HSE and HSE funded voluntary services
- Improved patient safety through the efficient handling of notices and alerts
- Availability of statistical and management reports
- Ambulance Arrivals Project A Case Study
- SNOMED National Release Centre (NRC)
- SVUH Award winning Patient Flow Whiteboard
- Scan for Surgery
- Hospital-based care
- Digital Natives Sign App
- Digitisation of risk assesment tools for Adult mental health services in north Dublin
- Patient Engagement Operating Systems - Hep C
- Digital Transition for HSCPs at St. James's Hospital
- Primary Care Centre Castlebar Case Study
- Mario - Managing active and healthy ageing using caring service robots
- Claimsure - Health Insurance Claims Management System
- Cyber Attack Response
- Data systems in SVUH Emergency Department
- Electronic Discharge Prescription Pilot
- Epilepsy EPR
- eReferral
- eReferral Radiology Pilot
- eRostering
- Electronic Blood Tracking
- GP Practice Management Systems
- Healthmail
- Heart Failure Virtual Clinic
- Infrastructure - MPUP to ECAM
- IT Security - Small changes, big difference
- Kidney Disease Clinical Patient Management System
- Local Asset Mapping Project at St James' Hospital
- LUCY
- Mi Kidney App
- Model Community
- NCHD - Employment Record Portal
- Nursing & Midwifery Quality Care Metrics
- Ophthalmology Electronic Patient Record
- PharmaBuddy
- Radiology & Electronic Patient Record
- National Smart-Pump Drug Library of Paediatric and Neonatal Standardised Concentration Infusions
- Quality & Patient Safety
- Robotic Assisted Surgery Programme
- Shared Learning on EHR
- St. James' Hospital - National Haemophilia System
- Tallaght Hospital Pharmacy
- Tallaght Hospital Patient Engagement App
- Track & Trace
- Using IT to Improve Ireland's Public Sector Healthcare
- National Audiology Clinical Management System (NA-CMS)
- St Vincent's University Hospital Award Winning Whiteboard Patient Flow System
- Snomed Case Study
- Telehealth Project Donegal
- St Vincent's Whiteboard Patient Journey System a Case Study
- Ambulance Arrivals Project
