
Update from the Laboratory Programme
The Laboratory Programme, under the governance of eHealth, was established in 2021 to advance, standardise and support key Laboratory ICT initiatives. The aim of this Programme is to provide a framework for laboratory services to engage with HSE eHealth & Acute Operations.
eOrdering for GPs:
The Laboratory Programme was delighted to welcome Ms. Mary Foley to the team in February. Mary has been appointed as the GP Workstream Lead and is working to implement GP eOrdering. It is great to have a dedicated resource on the team to manage this project. In latest developments; the display of orders within Healthlink is to be refined following a meeting with the GPIT group; additions to the GP catalogue are being referred to the NCPP for review; the job of mapping of orders and specimen types for the 3 pilot sites continues with an expectation of a Go-Live in Q4 this year.
LIS & hardware stabilisation of current LIS systems:
The Laboratory Programme team continue to support and advance projects to stabilise existing LIMS and associated hardware. In total, 11 LIMS sites have been upgraded and stabilised in the past year.
Examples of recent activities include:
Project ICT22067 Upgrade of servers is complete for St. Colmcilles, Connolly and Naas hospitals who were previously hosted on a shared alpha server. All sites are now live on 3 new virtual servers providing Telepath LIMS server stabilisation and resilience for the next 5-7 years.
- Project ICT23013 Upgrade of technical platform for servers/memory/cache supporting LIMS for Galway (GUH), Mayo University Hospital (MUH) and Roscommon University Hospital (RUH).
- Strategy for APEX hardware upgrade and technical refresh: The APEX Laboratory Information Systems (LIMS) is in operation at multiple sites across the state, some originally installed late 1990s and early 2000s. The server infrastructure and storage (SAN) at these sites is aged and a risk to laboratory services. The hardware requires a refresh which should be aligned with an application upgrade where applicable. It is proposed to continue the process of a full refresh on a phased basis over the next 4 years.
Procurement of a Compliance Management Information System (CMIS):
The Laboratory Programme procurement process for a compliance management information system (Project ICT22032) is progressing. In order to address contract life and meet customers need for this solution, HSE Procurement are using a procurement procedure called a Dynamic Purchasing System (DPS). A DPS differs from typical contract or frameworks in that the duration of the contract is not defined, multiple vendors can be included and there is the option to add new vendors during the lifetime of the DPS. The CMIS DPS was published on eTenders in April 2023. The HSE Procurement team are currently performing a qualification of all responses at which point scoring of the tender can begin. Once the contract is awarded, a fully cloud-hosted CMIS system will be implemented in the pilot site. This DPS contract will remain in place for subsequent sites to engage with.
MedLIS Progress:
Following the successful copy of the database to new servers in Sweden and the transition to RHO, connectivity was established with the various MedLIS local project offices and into Beaumont Hospital, our pilot site. As per the project plan, a comprehensive 12 week Change Build window then took place to update the build with the latest requirements for Beaumont lab including Core build of Consultants, PAS locations, GPs and GP practices, Order Catalogue changes and any new workflow changes. This window concluded on June 30th with 193 Change Requests completed and tested.
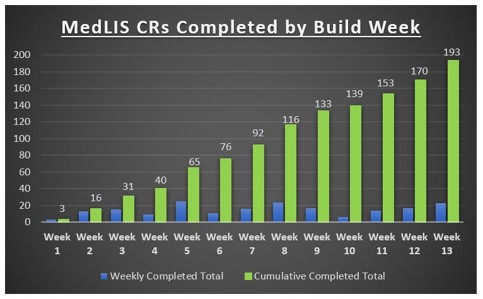
Currently the National Project Team are undergoing an 8-week Operational Qualification (OQ) Test Cycle. The MedLIS OQ involves a comprehensive functionality and build stability testing of the system using a suite of test scripts. Once NPT OQ is completed, the system is handed over to the Beaumont Lab team to continue testing with a full sit executed System Testing activity (Site OQ). Site OQ includes device interface testing, Foreign System integration testing and Integration Testing of scenarios for local workflows. This will inform their Change Management plan in preparation for Go-Live in May 2024.
Tracking batch products using GS1 barcodes – a first for Irish Blood Transfusion Laboratories:
MedLIS has exciting new functionality for Blood Transfusion. MedLIS will be the first L.I.S. to have a solution for batch products using GS1 barcodes. (All batch products now have GS1 barcodes to comply with the Falsified Medicines Directive.) Batch products will now be entered into stock with one easy scan.
For more information on The Laboratory Programme click here

